DAXXIFY
Conveniently located to serve the areas of New York, NY

DAXXIFY | NOW AVAILABLE
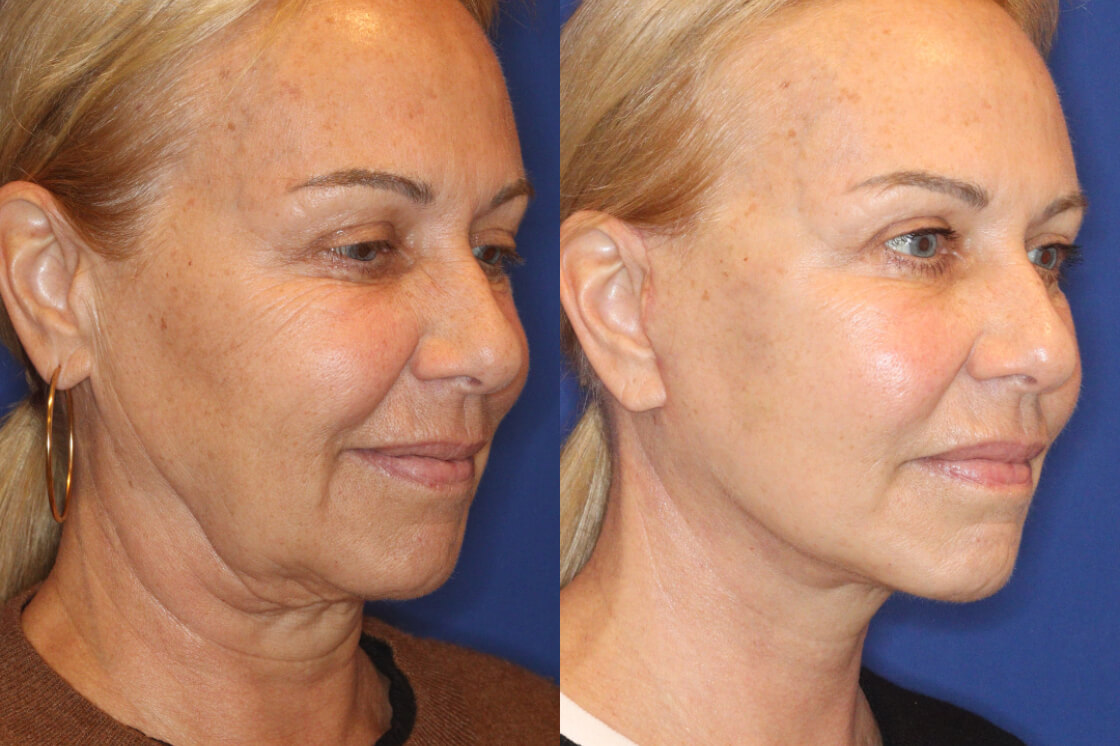
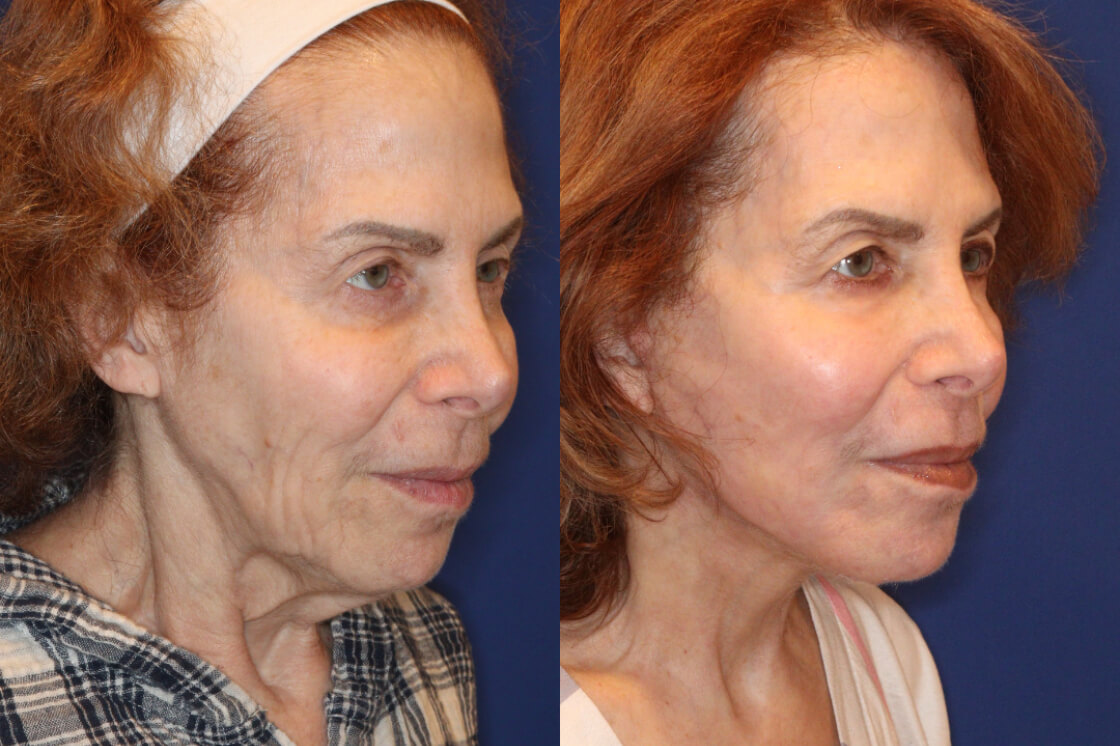
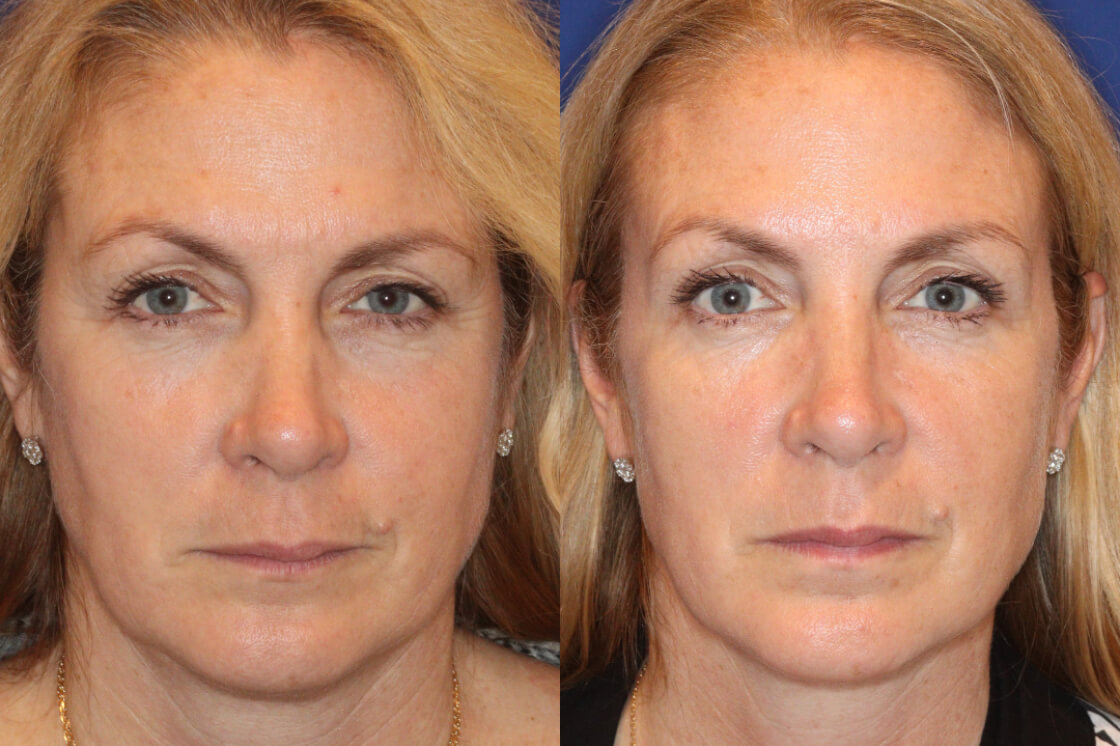
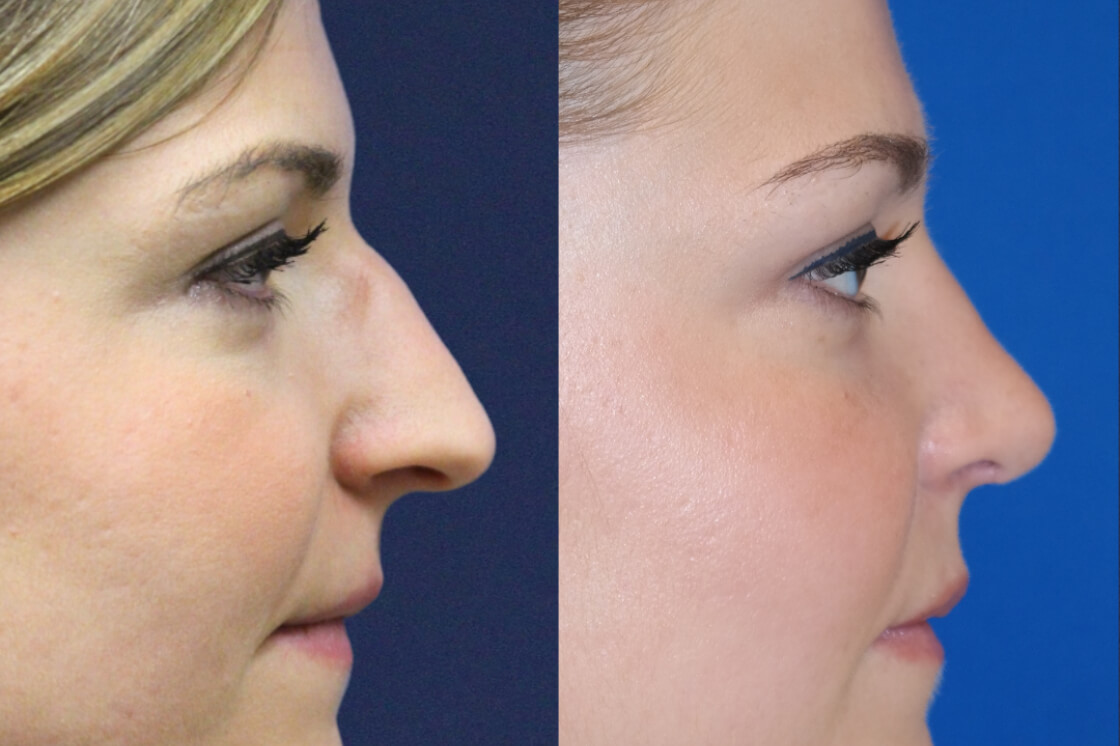
Daxxify is the first and only peptide-powered, FDA-approved frown line treatment designed to minimize the appearance of facial wrinkles, with a median duration of 6 months and up to 9 months for some patients.
Dr. Jennifer Levine is one the first to offer Daxxify in New York City.
Book NowContents
In The Media

As top 1 % injector in the country, Dr. Levine has been selected as one of the first providers in New York City to offer DAXXIFY.
Daxxify vs. Botox: NYC’s Dr. Jennifer Levine sheds light on the New Anti-wrinkle Injectable
What Is DAXXIFY?

Daxxify was developed by the manufacturer Revance Therapeutics. Studies showed the drug can temporarily improve “moderate to severe frown lines” in adults for a median duration of six months, which is up to two months longer than the period generally provided by Botox.
DAXXIFY Vs Botox: How Does It Work?
Like Botox, Daxxify is an acetylcholine release inhibitor and neuromuscular blocking agent. This means blocking certain chemical signals from nerves, mostly signals that cause muscles to contract, and temporarily relaxing the facial muscles that cause wrinkles.
However, Daxxify uses peptide exchange technology, which allows it to attach to the receptor more effectively and also accounts for the longer-lasting results. Currently, Daxxify is also the only neuromodulator that is free from human serum albumin, a protein found mostly in human blood or animal-based components.
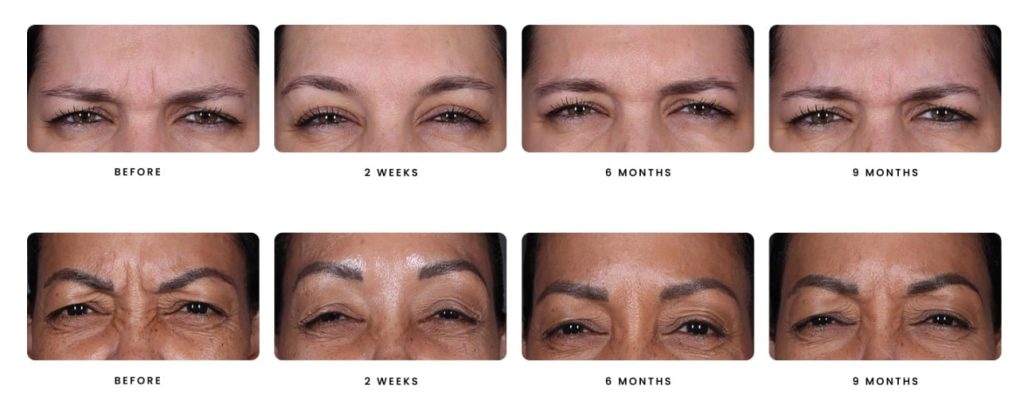
Whereas Botox can last approximately 3 – 4 months, Daxxify has shown to last up to 9 months with the median duration of 6 months – this is great news for our patients!
What Conditions Can DAXXIFY Treat?
In addition to reducing the appearance of fine lines and wrinkles, Daxxify can also used to treat conditions such as neck spasms, excessive sweating, an overactive bladder, and lazy eye.
Daxxify was found to be “generally safe and well tolerated” with no serious treatment-related adverse events reported in during the clinical trials.
FAQ
Contraindications
DAXXIFY™ contraindications include hypersensitivity to any botulinum toxin preparation or any of the components in the formulation and infection at the injection site(s).
Warnings and Precautions
Please refer to Boxed Warning for Distant Spread of Toxin Effect.The potency Units of DAXXIFY™ are not interchangeable with other preparations of other botulinum toxin products. Recommended dose and frequency of administration should not be exceeded. Patients should seek immediate medical attention if respiratory, speech or swallowing difficulties occur. Use caution when administering to patients with pre-existing cardiovascular disease. Concomitant neuromuscular disorders may exacerbate clinical effects of treatment.
Adverse Reactions
The most commonly observed adverse reactions (≥1%) were headache (6%), eyelid ptosis (2%) and facial paresis (1%).
Drug Interactions
Co-administration of DAXXIFY™ and aminoglycoside antibiotics, anticholinergic agents or any other agents interferinCo-administration of DAXXIFY™ and aminoglycoside antibiotics, anticholinergic agents or any other agents interfering with neuromuscular transmission or muscle relaxants should only be performed with caution as the effect of DAXXIFY™ may be potentiated. The effect of administering different botulinum neurotoxins during course of treatment with DAXXIFY™ is unknown.
Use in Specific Populations
DAXXIFY™ is not recommended for use in children or pregnant women.Please see DAXXIFY™ full Prescribing Information, including Boxed Warning and Medication Guide.
References
– DAXXIFY™. Prescribing Information. Revance Therapeutics, Inc, 2022.
– Fabi SG, Cohen JL, Green LJ, et al. DaxibotulinumtoxinA for Injection for the treatment of glabellar lines: efficacy results from SAKURA 3, a large, open-label, phase 3 safety study. Dermatol Surg. 2021;47(1):48-54.
– BOTOX® Cosmetic. Prescribing Information. Allergan Inc; 2020.
– XEOMIN®. Prescribing Information. Merz Pharmaceuticals GmbH; 2021.
– DYSPORT®. Prescribing Information. Ipsen Biopharm Ltd; 2020.
– JEUVEAU®. Prescribing Information. Evolus, Inc; 2020.
– Carruthers JD, Fagien S, Joseph JH, et al. DaxibotulinumtoxinA for Injection for the treatment of glabellar lines: results from each of two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). Plast Reconstr Surg. 2020;145(1):45-58.
– Data on File. Protocols 1620301-303. Post Hoc Analysis. Newark, CA: Revance Therapeutics, Inc, 2021.
– Bertucci V, Solish N, Kaufman-Janette J, et al. DaxibotulinumtoxinA for Injection has a prolonged duration of response in the treatment of glabellar lines: pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). J Am Acad Dermatol. 2020;82(4):838-845.